Translational Research in Veterinary Science Vol. 6 No. 2 (2023) p. 43 – 57
1Mencel Jakub, 1Osten-Sacken Natalia
1Institute of Veterinary Medicine, Faculty of Biological and Veterinary Sciences
Nicolaus Copernicus University in Toruń
mencel.jakub16@gmail.com
Abstract
The role of bats in nature is undoubtedly significant. A huge contribution to seed dispersal (fruit bats) and insect reduction (carnivorous bats) have a very important impact on the ecosystem balance around the world. In addition, bats are primarily associated with being vectors for a number of diseases of global importance.
Given the obvious impact of bats on the balance of ecosystems and the health of animals, including humans, it seems that a thorough understanding of the biology of these mammals has a great value to the life of the entire planet. Research on the parasitic fauna of bats is certainly of particular importance – understanding the morphology of external and internal parasites, their physiology, life cycles and the possible consequences of the presence in the bat’s body. This knowledge is not only helpful in increasing the effectiveness of the protection of these mammals but also makes it possible to predict the effects on the health and life of animals and humans, whose ecosystems are increasingly intertwined with bat roosts, which closely aligns with the popular and integrated One Health approach.
The main objective of this study was to demonstrate whether there are differences in the structure and type of parasites of bat’s in their maternity colonies, depending on the species and geographic region of Poland.
Key words: bats, internal parasites, external parasites, maternity colonies, public health care.
Introduction
Bats belong to the order Chiroptera, which represents more than 1300 species described so far, making them the second largest (after rodents) order of mammals [1]. According to current data, there are 27 species of bats in Poland. These are 2 species from the Rhinolophidae family, 24 species from the Vespertilionidae family and recently found for the first time in the Rożnów Foothills – Schreibers’ bat (Miniopterus schreibersii), the only representative of the Miniopteridae [2].
All Polish species are insectivorous, which effectively reduces their number. Thanks to their peculiar features specific only to them, they have become excellent predators of insects. Nocturnal lifestyle, flight ability, high metabolic rate or the ability to use echolocation have made bats potentially perform a very important function as a biological factor in the control of pests from the order of scaly-winged (Lepidoptera), as well as some species of flies (Diptera) and beetles (Coleoptera) [3].
In addition, there are species in the world for which use fruits and flower’s nectar as nourishment, which effectively contribute to seed spreading and pollination of plants, often of great economic importance.
Bats also act as one of the most important links in the epidemiological chain. They are a reservoir and often vectors in the transmission of many pathogens. They are responsible for spreading rabies virus, Nipah and Hendra, filovirus diseases (Ebola and Marburg), severe acute respiratory distress syndrome (SARS) and Middle East respiratory distress syndrome (MERS), among others. In addition, it is possible for bat viruses to cross the interspecific barrier, including humans, directly (rabies) or indirectly (Nipah and Hendra) by other animal species [4].
Bats are also hosts for a broad spectrum of endo- and ectoparasites, which can be vectors for the viruses. The parasitic fauna of bats, like all wild animals, shows a very large variety.
An unusual feature of bats is also their ability to easily synurbanize and synatropize, unparalleled among other free-living animals. On the other hand, people moving to previously uninhabited, original areas cause disruption of the natural balance of the ecosystem, which in turn leads to increased contact with microorganisms of wild animals [5].
Both of these sides mean that the probability of direct contact in the relation between bats and another animals also humans is higher then ever.
Methods
The assessment of parasite diversity was made on the basis of the analysis of guano samples collected in bat maternity colonies in the Polish area, after obtaining the consent of the Regional Directorates for Environmental Protection of the relevant voivodships.
Guano samples were collected with sterile nitrile gloves and placed in eppendorf tubes containing a 70% ethanol solution.
Depending on the size of the breeding colony:
Small colonies – 1 tube of eppendorf from the central point of the breeding colony.
Medium colonies – 2 tubes from the two extreme points of the colony.
Large colonies – 3 tubes from two extreme points and the central point.
For the study, all samples from one colony were mixed.
The detailed location of the maternity colonies where the samples were taken and the species of bats inhabiting them is shown in Table 1 and Map 1.
Table 1. Location of maternity colonies where samples were taken and bat species inhabiting them:
| Locality | Voivodeship | Bat species |
| Brenna | Silesian | Rhinolophus hipposideros |
| PN Bory Tucholskie | Pomeranian | Myotis dasycneme |
| Ojcowski PN Villa „Urocza” | Lesser Poland voivodeship | Rhinolophus hipposideros |
| Ojcowski PN Villa „Jadwiga” | Lesser Poland voivodeship | Rhinolophus hipposideros |
| Kiszewo | Greater Poland | Myotis myotis |
| Forest | Lower silesian voivodeship | Myotis myotis |
| Pilaszkowice | Lublin | Plecotus auritus |
| Rościszów | Lower silesian voivodeship | Myotis myotis |
| Rzuchowa | Lesser Poland voivodeship | Myotis myotis, Rhinolophus hipposideros |
| Sieraków | Greater Poland | Myotis myotis |
| Slawków | Silesian | Myotis emarginatus |
| Strączno | West Pomerania | Myotis myotis |
| Sulechów | Lubuskie | Myotis myotis |
| Poznan | Greater Poland | Nyctalus noctula |
Map 1. . Location of maternity colonies where samples were taken:
The guano samples were stored at -20°C until the start of the analysis.
The analysis of the variety of forms of endoparasites was made on the basis of parasitological examination under a microscope.
Results
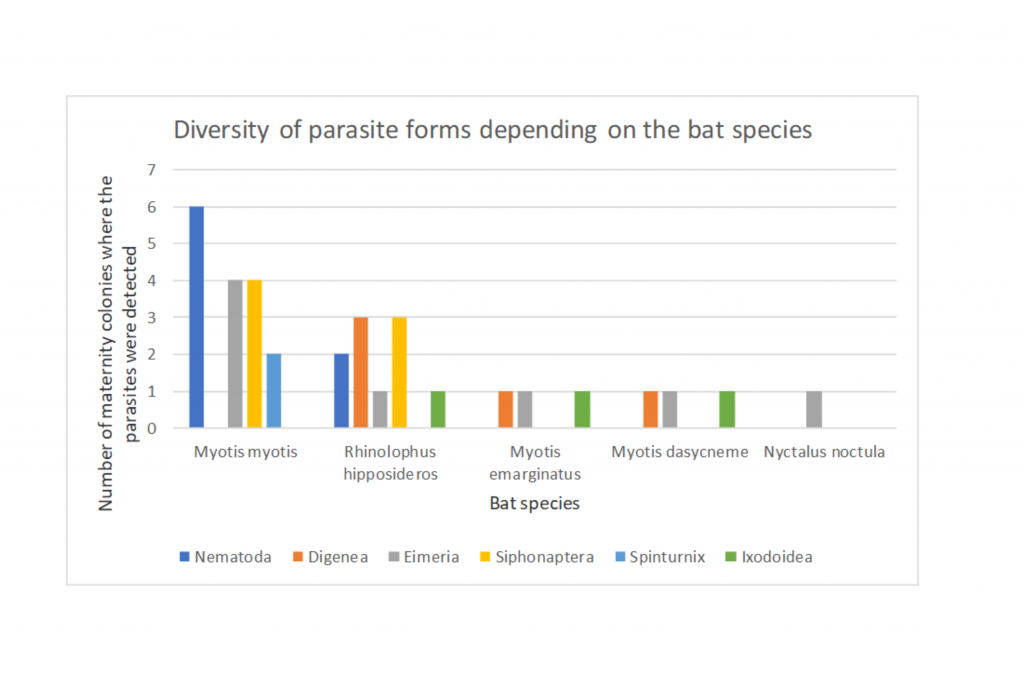
Parasitological examination under a microscope allowed to find in guano samples 6 taxes of parasites: 3 internal parasites and 3 ectoparasites.
Of the ectoparasites found in the form of eggs and larvae of ticks (Ixodoidea), fleas (Siphonaptera) and individuals of adult mites (Spinturnix spp.).
Of the internal parasites, the following were detected in the samples: eggs (Digenea) and nematode eggs (Nematoda) and protozoan oocysts (Eimeria spp.).
The location of the shelters, the species of bat and the type of parasite detected at the site are presented in Table 2.
Table 2 Location of the maternity colony, the species of bat and the type of parasite detected at the site:
| Location of the maternity colony | Bat species | Type of parasite | ||
| Villa „Jadwiga” Ojcowski PN | Rhinolophus hipposideros | Digenea, Eimeria sp., Siphonaptera, | ||
| Villa „Urocza” Ojcowski PN | Rhinolophus hipposideros | Nematoda | ||
| PN Bory Tucholskie | Myotis dacysneme | Digenea, Eimeria sp., Ixodoidea | ||
| Forest | Myotis myotis | Nematoda, Siphonaptera, Spinturnix | ||
| Sławków | Myotis emarginatus | Eimeria sp. Digenea, Ixodoidea | ||
| Rościszów | Myotis myotis | Nematoda, Eimeria sp., Siphonaptera | ||
| Brenna | Rhinolophus hipposideros | Digenea, Siphonaptera, | ||
| Sulechów | Myotis myotis | Eimeria sp., Nematoda, Spinturnix | ||
| Poznan | Nyctalus noctula | Eimeria sp. | ||
| Pilaszkowice | Plecotus auritus | – | ||
| Rzuchowa | Myotis myotis, Rhinolophus hipposideros | Nematoda, Digenea, Siphonaptera, Ixodoidea | ||
| Kiszewo church | Myotis myotis | Nematoda, Eimeria sp., Siphonaptera | ||
| Sieraków | Myotis myotis | Nematoda, Eimeria, | ||
| Strączno | Myotis myotis | Nematoda, Siphonaptera | ||
The variety of parasite forms depending on the bat species is shown in Figure 1.
Figure 1 The variety of parasite forms depending on the bat species:
Discussion
Due to colonial customs, bat’s females gather in the spring-summer period in large clusters called maternity colonies, in order to give birth and raise offspring. These colonies are most often established in warm attics of churches or residential buildings. In terms of reproduction, bats are very dependent on human.
Reproductive communities assume close contact between individuals, which is why bat organisms are often inhabited by many species of micro- and macroparasites moving from one individual to another.
In maternity colonies, ectoparasites and internal parasites have an excellent opportunity to spread not only to other females, but above all to their offspring, because it is a place of constant tactile contact in the relationship between female-female and mother-offspring.
These two relationships, on which the maternity colony is based, perfectly show that it is a place where the extensiveness and intensity of parasite invasion increase significantly and are the largest in the entire annual cycle of the bat.
All the colonies included in this study were located in the attics of buildings, one colony in the basement.
It was shown that the most abundant ectoparasite were fleas. They were identified after large amounts of eggs and larval forms were detected in guano. The flea develops in several stages, starting from the egg, then into the stage of the larva, pupa and finally in the adult stage.
A flea can complete its life cycle as early as 14 days [6]. However, it usually lasts about three weeks. Adult individuals live in the coat and wings of bats and feed on the blood of their host. In general, fleas living on daytime hosts have well-developed eyes, while species parasitizing on underground hosts or nocturnal animals such as bats have poorly developed eyes or do not have them at all [7]. Eggs are laid on the skin and then fall from the hanging host to the bottom of the maternity colony. They grow in guano lying at the bottom of the maternity colony. The answer to such a great intensity of flea invasion is their very fast life cycle. Fleas acquire sexual maturity very early, and an adult flea during its life can lay up to two thousand eggs.
A popular ectoparasite of bats are also ticks mainly of the genus Argas and Ixodes. Caught bats are often affected by the massive invasion of these arachnids. Warm and dark maternity colonies are an ideal shelter for ticks, their eggs and larvae. At the beginning of summer, the female tick lays up to five thousand eggs, which is why they are found in large numbers in guano. Adult individuals are found there rather accidentally.
Among the ectoparasites, we also recognized mites of the genus Spinturnix, for which the habitat is mainly the volatile membranes of bats. They pass their entire life cycle on parts of the body equipped with membranes and feed on the blood of their host. They breed at a time when the female bat gives birth to her offspring, which makes it easier for the mite to spread. In addition, they are detritus eaters and the area of the reproductive colony abounds in organic matter in the form of guano. In the feces, the occurrence of mites of the genus Spinturnix is found in large numbers, on the basis of which it can be assessed that the intensity of invasion in the colony is significant.
In the study, the most numerous group were the maternity colonies of the Greater mouse-eared bat (Myotis myotis) and the Lesser horseshoe bat (Rhinolophus hipposideros).
It happens that both of these species occupy a common area, and are even found in one colony, e.g. in Rzuchowa and Brenna, but they prefer slightly different types of feeding grounds.
Greater mouse-eared bat can be found mainly in densely forested areas in western and central, but also partly southern Poland. The Lesser horseshoe bat has a slightly narrower area of occurrence. It is found in the Kraków-Częstochowa Upland, in the Eastern Sudetes and in the limited area of the Carpathians [8]. It prefers warmer, rocky places, often near water. The area of occurrence of the Lesser horseshoe bat in the south of Poland, stretching from the Lower Silesian Voivodeship to the Podkarpackie Voivodeship is called the Horseshoe Land.
In guano of the Greater mouse-eared bat, digenetic flukes (Digenea) were completely absent, while a very high number of nematode eggs (Nematoda) were found. In a study conducted in Germany in the breeding colony of the Greater mouse-eared bat in Gladenbach, the situation was very similar. Six species of insect and arachnid ectoparasites were detected, namely Ixodes ricinus, Ischnopsyllus octactenus, Ichoronyssus scutatus, Steatonyssus periblepharus, Spinturnix myoti and Cimex dissimilis, as well as the nematode Molinostrongylus alatus. Digenea, on the other hand, was not found [9].
It turns out that the direct impact on the diversity of internal parasites is not only about the species or environment itself, but the hunting strategy and the type of diet. The strategy of hunting the Greater mouse-eared bat involves searching for insects on the ground and landing directly on its potential victims. The Greater mouse-eared bat, thanks to the large fragments of the remains of its victims in guano, is very well known in terms of food preferences. In the feces of the Greater mouse-eared bat, ground arthropods are most often found, primarily runners, among which runners of the genus Carabus have a very large share. In addition, the diet of the Greater mouse-eared bat includes wriggles (Myriapoda), spiders (Araneae) and larvae of beetles (Coleoptera) [10]. According to the results of research conducted by Graclik and Wasielewski Coleoptera were most abundant in fecal samples in all seasons [11].
In the case of samples from a Lesser horseshoe bat, the situation was quite the opposite. In guano of this species, a significant amount of digenetic fluke eggs and insignificant amounts of nematode eggs were found. Studies conducted in Spain in the maternity colonies of the Greater horseshoe bat, a species also found in Poland and very close to the Lesser horseshoe bat, have shown that infestations of Trematoda flukes, especially Digenea, are characteristic of the horseshoe bat family and closely related to their diet [12]. The Lesser horseshoe bat hunt strategy assumes scanning the environment from the shelter and hunting on the fly for potential victims. Hence, in his diet there are slightly different insects than in the diet of the Greater mouse-eared bat. In guano studies to determine the nutritional composition of the Lesser horseshoe bat carried out in Germany, therefore an area similar in climate to Poland, the remains of flies (Diptera), scaly-winged (Lepidoptera) and networms (Neuroptera) [13] were found in the droppings. Crane flies (Tipula oleracea) was the most common. In addition to them, Hymenoptera, Chrysopidae and Culicoidea [14] were found in large quantities. It seems that the reason for such a diet of Lesser horseshoes bats should be found in the distance of their summer hiding places from water, which are an important component of their feeding grounds.
It is a known fact that the development cycle of flukes is based on the aquatic ecosystem and the organisms inhabiting it. In addition, to complete their cycle, flukes can cross environmental barriers and move from aquatic hosts to terrestrial hosts feeding on them, such as amphibians, birds, mammals and in particular bats [15]. Fluke eggs enter the water along with an external factor, which can be, for example, bat guano, which hunts over a body of water. Then they go to the body of a water snail, in which they reach the next stage of development and as cercariae change the host to the larval stage of insects, whose development cycle takes place in the aquatic environment, e.g. mosquitoes. With the transformation of the larval form of an insect into an imago, cercariae transform into metacercaria. If an insect in which metacercariae occur falls prey to a bat, then it becomes the ultimate host for flukes, which reach an adult form and can produce eggs. This is how the whole cycle closes.
In addition, bat guano is also abundantly found in protozoan oocysts of the genus Eimeria spp, but without significant differences. The invasion of these protozoa is characteristic of wild animals, including bats.

Females of Lesser horseshoe bat and Greater eared-mouse bat sharing one maternity colony in Brenna.
Conclusion
Our research based on the analysis of bat guano using a microscope showed that the parasitic fauna of bats is very diverse.
In the case of ectoparasites, the results obtained by us were very similar.
Ectoparasites do not show significant variability depending on the species and the environment. They are found in large numbers in each maternity colony, which are an excellent shelter for them, abundant in potential host organisms.
However when it comes to internal parasites, the results turned out to be surprising, especially in relation to the differences between the Greater mouse-eared bat and the Lesser horseshoe bat.
It seems that the environment in which bats live does not directly affect the composition of parasitic fauna, because in the south of Poland there are maternity colonies, where both species occur in one hiding place.
On the other hand, the way that individual species implement their hunting strategy, and which arthropods are in the diet of a particular bat species seems to have a direct impact on the diversity of the intracorporeal parasitic fauna of bats.
References
[1] Volleth M, Eick G. „Chromosome evolution in bats as revealed by FISH: the ongoing search for the ancestral chiropteran karyotype” Cytogenet Genome Res. 2012;137:165–73.
[2] Piksa K. , Gubała WJ. „The first mention of Miniopterus schreibersii (Chiroptera: Miniopteridae) in Poland – a possible extension of the range?”. Mamm Res 66, 211–215 (2021).
[3] Burgiełł P. „Bats as a potential biological pest control agent in forest” (2018) Sylwan 162(9):707-717
[4] Gliński Z. ,Ciołek J. „Bats as reservoirs and vectors of pathogenic viruses for man and animals” Veterinary Life 2020 ; 95(1)
[5] Smreczak M., Żmudziński J. „Bats and their behavior”, Medycyna Wet 2006; 62(9)
[6] Krämer F. Mencke N. (2001) Developmental Cycle of Fleas. In: Flea Biology and Control. Springer, Berlin, Heidelberg.
[7] Rothschild M. , Traub R. „flea”. Encyclopedia Britannica , (2022), https://www.britannica.com/animal/flea.
[8] Sachanowicz K., Ciechanowski M., Piksa K. 2006. „Distribution patterns, species richness and status of bats in Poland”. Vespertilio 9, 10: 151-173.
[9] Frank, R., Kuhn, T., Werblow, A. et al. Parasite diversity of European Myotis species with special emphasis on Myotis myotis (Microchiroptera, Vespertilionidae) from a typical nursery roost. Parasites Vectors 8, 101 (2015).
[10] Dietz Ch., von Helversen O., Nill D., „Bats of Europe and North-West Africa”, Multico Publishing House, (2009) pp.168-172.
[11] Graclik A., Wasielewski O. „Composition of the Myotis myotis diet in western Poland, Results of fecal analysis”; (2012) Turkish Journal of Zoology 36(2):209-213
[12] Esteban J.G., Botella P., Toledo R. & Oltra-Ferrero J.L., 1999.- Helminthfauna of bats in Spain. IV. Parasites of Rhinolophus ferrumequinum (Schreber, 1774) (Chiroptera: Rhinolophidae).
[13] Mitschunas N., Wagner M. „Diet of the Lesser Horseshoe Bat ( Rhinolophus hipposideros ) in Central Germany and Its Seasonal and Site-Specific Variation” (2015) Acta Chiropterologica 17(2):379-392
[14] Dietz Ch., von Helversen O., Nill D., „Bats of Europe and North-West Africa”, Multico Publishing House, (2009) pp.252-257.
[15] Niewiadomska K. , Pojmańska T. „Multiple strategies of digenean trematodes to complete their life cycles”; Wiadomości Parazytologiczne 2011, 57(4), 233–241